近日,中山大学中山医学院邓凯教授团队有关HIV-1潜伏储存库维持机制的研究取得新进展,该团队发现一类表达CD161表面分子的CD4+ T细胞亚群在HIV-1的潜伏感染中扮演重要角色,此细胞亚群的克隆扩增可能是维持了感染者体内病毒储存库的长期稳定的重要机制。该成果为从靶向病毒储存库的角度寻找到艾滋病功能性治愈新策略提供了新的思路,相关研究结果发表在微生物学权威学术期刊mBio上,题目为CD161+ CD4+ T Cells Harbor Clonally Expanded Replication-Competent HIV-1 in Antiretroviral Therapy-Suppressed Individuals。

艾滋病是一种由HIV-1感染引起在全球范围内造成极大危害的人类传染病。HIV-1攻击人体免疫系统的CD4阳性T淋巴细胞,使感染者逐步丧失免疫功能。尽管联合抗病毒治疗(鸡尾酒疗法)能有效控制HIV-1复制,极大延长了感染者的寿命,但由于病毒可潜伏感染形成稳定的储存库,HIV-1感染至今仍无法治愈。感染者只要停药,血液中的病毒含量即迅速反弹,这意味着一旦感染就面临着需要终身服药的残酷现实。
病毒储存库的长期存在是治愈艾滋病的主要障碍,而HIV-1潜伏感染的细胞发生克隆增殖是病毒储存库在体内长期稳定存在的关键因素之一。然而,驱动潜伏感染细胞发生克隆增殖的机制仍不明确,具有特定生物学特征的细胞亚群在储存库克隆扩增中所起的作用也未有报道。
本研究首先在体外实验中发现CD161+ CD4+ T细胞对HIV-1高度易感,呈现记忆性CD4+ T细胞的特征,并具有典型滤泡辅助性T细胞(TFH)及Th17细胞的表型。与不表达CD161的CD4+ T细胞相比,该细胞亚群表现出更强的存活能力和细胞因子(IL-7和IL-15)驱动的稳态增殖能力。在此基础上,邓凯团队与广州市第八人民医院蔡卫平、李凌华临床团队合作,发现在接受抗病毒有效治疗的感染者中,CD161+ CD4+ T细胞含有更高频率的复制全能性的潜伏病毒。进一步通过对分离得到的潜伏病毒进行单克隆测序及进化关系分析,证实CD161+ CD4+ T细胞比CD161- CD4+ T细胞含有更多的完全相同的前病毒序列,提示病毒感染的该群细胞在体内发生了克隆扩增,促进了病毒储存库的增殖。
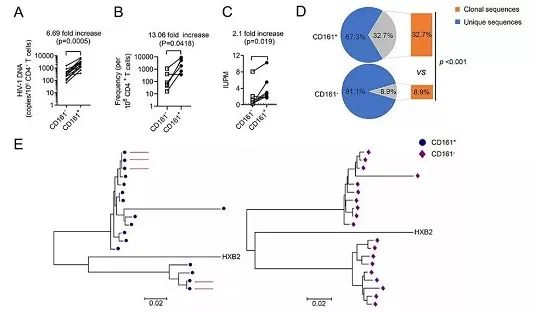
Figure CD161+ CD4+ T cells harbor more replication-competent latent HIV-1 and clonal expanded proviruses than CD161- CD4- T cells.
本研究提出了抗病毒治疗下储存库细胞扩增的新机制,并鉴定了介导该过程的CD4+ T细胞亚群,为后期靶向抑制HIV-1储存库克隆增殖的新手段提供了理论基础。我院李晓敏博士是该论文的第一作者,邓凯教授、蔡卫平主任及李凌华主任是论文的共同通讯作者。该研究受到国家科技重大专项、国家自然科学基金、广东省青年珠江学者项目和广东省杰出青年科学基金的资助。
ABSTRACTThe presence of an extremely stable latent reservoir of HIV-1 is the major obstacle to eradication, despite effective antiretroviral therapy (ART). Recent studies have shown that clonal expansion of latently infected cells without viral reactivation is an important phenomenon that maintains the long-term stability of the reservoir, yet its underlying mechanism remains unclear. Here we report that a subset of CD4+ T cells, characterized by CD161 expression on the surface, is highly permissive for HIV-1 infection. These cells possess a significantly higher survival and proliferative capacity than their CD161-negative counterparts. More importantly, we found that these cells harbor HIV-1 DNA and replication-competent latent viruses at a significantly higher frequency. By using massive single-genome proviral sequencing from ART-suppressed individuals, we confirm that CD161+ CD4+ T cells contain remarkably more identical proviral sequences, indicating clonal expansion of the viral genome in these cells. Taking the results together, our study identifies infected CD161+ CD4+ T cells to be a critical force driving the clonal expansion of the HIV-1 latent reservoir, providing a novel mechanism for the long-term stability of HIV-1 latency.
IMPORTANCE The latent reservoir continues to be the major obstacle to curing HIV-1 infection. The clonal expansion of latently infected cells adds another layer maintaining the long-term stability of the reservoir, but its mechanism remains unclear. Here, we report that CD161+ CD4+T cells serve as an important compartment of the HIV-1 latent reservoir and contain a significant amount of clonally expanded proviruses. In our study, we describe a feasible strategy that may reduce the size of the latent reservoir to a certain extent by counterbalancing the repopulation and dissemination of latently infected cells.